Fat and oil processing
Fat and oil processing, method by which animal and plant substances are prepared for eating by humans.
The oil and fat products used for edible purposes can be divided into two distinct classes: liquid oils, such as olive oil, peanut oil, soybean oil, or sunflower oil; and plastic fats, such as lard, shortening, butter, and margarine. The physical nature of the fatty material is unimportant for some uses, but the consistency is a matter of consequence for other products. As a dressing on green salads, for example, a liquid oil is used to provide a coating on the ingredients; a plastic fat such as lard or butter would be unsuitable. Spreads for bread, foods that require a highly developed dough structure, or icings and fillings with a plastic structure require plastic fats rather than liquid oils.
For reasons related to both history and climate, there are pronounced geographic patterns of consumption of fats and oils. The ancestors of the present inhabitants of central and northern Europe obtained their edible fats almost exclusively from domestic animals. The food habits and the cuisine depended on the availability of plastic fats; and butter, lard, margarine, and shortening continue to be their primary fatty food materials. In contrast, population pressures in the older civilizations of the Orient and the Mediterranean countries of southern Europe, northern Africa, and the Middle East have long since made extensive raising of livestock impractical, necessitating that the edible oils of these regions be derived primarily from intensively cultivated vegetable crops. In the tropics, conditions are relatively unfavourable for livestock but are well suited to culture of a variety of oil-bearing plants, many of which flourish in the wild state. In contrast to most high-population-density tropical areas, cattle abound in India. Clarified butter or ghee is an important item of Indian cookery, and a hydrogenated shortening called vanaspati is designed to reproduce the coarsely crystalline plastic texture of ghee.
More than 90 percent of the world production of fats and oils is used in edible products, and the objective of most processing steps is to convert crude fats of low palatability or undesirable physical form into refined products that meet the regional requirements for food fats. The annual consumption of visible fats—such as lard, butter, shortening, or salad oils that have been separated from the original animal or plant source—ranges from 18 to 25 kilograms (40 to 55 pounds) per person in various highly industrialized European countries to 23 kilograms per person in the United States. For the world as a whole, the average available supply is 10 kilograms per person; and in many areas of South America, Africa, and Southeast Asia, the annual consumption is 5 kilograms or less per person.
About 40 percent of the dietary fat in the developed countries comes from isolated fats and oils, with 60 percent obtained from basic foods, whereas in the less developed countries most of the dietary fat is obtained from fruits, cereals, vegetables, dairy products, and meats, and relatively little is consumed in the form of isolated fat products. The quantities of fats and oils in conventional food supplies vary over wide ranges. Most fruits and vegetables have from 0.1 to 2.0 percent fat, with the exception of avocados and olives, which are exceptional in their high fat content. Cereals range from 1 to 7 percent, and nuts may contain as much as 70 percent fat.
General Methods Of Extraction
The raw materials for the fat and oil industry are animal by-products from the slaughter of cattle, hogs, and sheep; fatty fish and marine mammals; a few fleshy fruits (palm and olive); and various oilseeds. Most oilseeds are grown specifically for processing to oils and protein meals, but several important vegetable oils are obtained from by-product raw materials. Cottonseed is a by-product of cotton grown for fibre, and corn oil is obtained from the corn germ that accumulates from the corn-milling industry, whose primary products are corn grits, starch, and syrup.
Fats may be recovered from oil-bearing tissues by three general methods, with varying degrees of mechanical simplicity: (1) rendering, (2) pressing with mechanical presses, and (3) extracting with volatile solvents. (See Figure 1.)
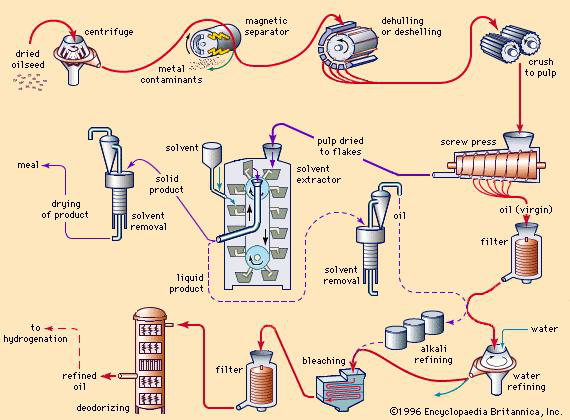
Figure 1
Rendering
Fruits and seeds
The crudest method of rendering oil from oleaginous fruits, still practiced in some countries, consists of heaping them in piles, exposing them to the sun, and collecting the oil that exudes. In a somewhat improved form, this process is used in the preparation of palm oil; the fresh palm fruits are boiled in water, and the oil is skimmed from the surface. Such processes can be used only with seeds or fruits (such as olive and palm) that contain large quantities of easily released fatty matter.
Animal fats
The rendering process is applied on a large scale to the production of animal fats such as tallow, lard, bone fat, and whale oil. It consists of cutting or chopping the fatty tissue into small pieces that are boiled in open vats or cooked in steam digesters. The fat, gradually liberated from the cells, floats to the surface of the water, where it is collected by skimming. The membranous matter (greaves) is separated from the aqueous (gluey) phase by pressing in hydraulic or screw presses; additional fat is thereby obtained. The residue is used for animal feed or fertilizer. Several centrifugal separation processes were developed in the 1960s. Cells of the fatty tissues are ruptured in special disintegrators under close temperature control. The protein tissue is separated from the liquid phase in a desludging type of centrifuge, following which a second centrifuge separates the fat from the aqueous protein layer. Compared with conventional rendering, the centrifugal methods provide a higher yield of better-quality fat, and the separated protein has potential as an edible meat product.
Pressing
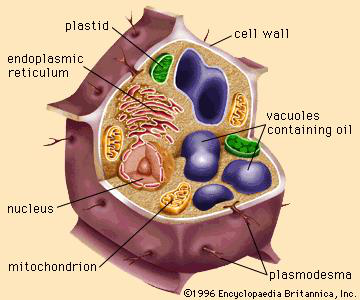
With many oil-bearing seeds and nuts, rendering will not liberate the oil from the cellular structures in which it is held (see Figure 2). In these cases the cell walls are broken by grinding, flaking, rolling, or pressing under high pressures to liberate the oil. The general sequence of modern operations in pressing oilseeds and nuts is as follows: (1) the seeds are passed over magnetic separators to remove any stray bits of metal; (2) if necessary, the shells or hulls are removed; (3) the kernels or meats are converted to coarse meal by grinding them between grooved rollers or with special types of hammer mills; and (4) they are pressed in hydraulic or screw presses with or without preliminary heating, depending on the type of oil-bearing material and the quality of oil desired. Oil expressed without heating contains the least amount of impurities and is often of edible quality without refining or further processing. Such oils are known as cold-drawn, cold-pressed, or virgin oils. Pressing the coarse meal while it is heated removes more oil and also greater quantities of nonglyceride impurities such as phospholipids, colour bodies, and unsaponifiable matter. Such oil is more highly coloured than cold-pressed oils. Residual meals are concentrated sources of high-quality protein and are generally used in animal feeds.
Figure 2: Some of the structures of an oilseed cell, including oil-containing vacuoles.
Many different mechanical devices have been used for pressing. The Romans developed a screw press, described by Pliny, for the production of olive oil. Centuries ago, the Chinese employed the same series of operations followed in modern pressing mills—namely, bruising or grinding the seeds in stone mills, heating the meal in open pans, and then pressing out the oil in a wedge press. The Dutch, or stamper, press invented in the 17th century was used almost exclusively in Europe for pressing oilseeds until the early part of the 19th century, when the hydraulic press was developed. The yield of oil from the hydraulic press was considerably higher than that from earlier processing methods because of the much higher applied pressures. In open presses, the ground seed material was confined in cloths of human hair or, less commonly, camel hair. Pressures on the cake varied from approximately 70 to 140 kilograms per square centimetre (1,000 to 2,000 pounds per square inch), and in the closed-type press, in which the oil-containing material was confined in a strong perforated steel cage during the pressing operation, pressures of approximately 400 kilograms per square centimetre or more were attained. Under ideal conditions the oil content of the hydraulic-press cake can be reduced to about 3 percent, but in practical operation a 5 percent level is average. The modern screw press replaced many of the hydraulic presses because it is a continuous process, has greater capacity, requires less labour, and will generally remove more oil. As ground seed is fed continuously into the mechanical press, a worm screw increases the pressure progressively as the material moves through a slotted barrel. Pressures from 700 to 2,100 kilograms per square centimetre are attained, and the oil is squeezed out through the slots, leaving a cake containing 3 to 3.5 percent oil under optimum processing and 4 to 5 percent oil under average conditions.
Solvent extraction Processes
Cakes obtained by pressing operations still retain 3 to 15 percent of residual oil. When the value of the oil is considerably greater as oil than as a part of the meal, it is desirable to obtain more complete extraction with solvents. Modern commercial methods of solvent extraction use volatile purified hydrocarbons, especially the various grades of petroleum benzin (commonly known as petroleum ether, commercial hexane, or heptane). In large-scale operations, solvent extraction is a more economical means of recovering oil than is mechanical pressing. In the United States and increasingly in Europe, there are many instances of simple petroleum benzin extraction of seeds, mainly soybeans. For seeds or nuts containing a higher oil content than soybeans it became customary to press the material in screw presses to remove a large proportion of the oil before extraction. Since this prepressing also ruptures the cellular structures of oil-bearing materials, most of the residual oil is easily removed with solvents.
A typical extraction system consists of (1) cleaning to remove tramp iron, dirt, foreign weed seeds, and stones, (2) removing hulls or cortex in cracking, aspirating, or screening operations, (3) cracking or rough grinding the kernels, meats, or prepressed cake, (4) steaming (tempering or cooking) of the meats, (5) flaking the small pieces between smooth flaking rolls, (6) extracting the oil with solvent, (7) separating the meal, or marc, from the oil-solvent solution, called miscella, and (8) removing the solvent from both the miscella and the marc. The marc may be toasted or pelletized, or both, for use in animal feeds. Most extracted meals contain less than 1 percent of residual oil. The amount varies depending on the amount of prepressing, the type of material being extracted, and the efficiency of the extracting system.
Extractors
Solvent extraction was first practiced in Europe, using batch extractors for the recovery of additional oil from the residues obtained from mechanical pressing. The greater efficiency of solvent extraction encouraged direct application to oilseeds, and the batch extractor gradually gave way to continuous units in which fresh flakes are added continuously and subjected to a counterflow of solvent. One of the earliest continuous extractors, and a type still considered to be one of the best, was the Bollman or Hansa-Mühle unit from Germany, in which solvent percolates through oilseed flakes contained in perforated baskets moving on an endless chain. After the extraction cycle is complete, the baskets of extracted flakes are dumped automatically and then refilled with fresh flakes to initiate another cycle. Many extractor designs have been proposed, but only a few have found wide acceptance. In the DeSmet extractor, popular in Europe and in a number of developing countries, a bed of flakes on an endless horizontal traveling belt is extracted by solvent percolation. The Blaw-Knox Rotocell has become the most popular extractor in the huge American soybean industry. The flakes are conveyed into wedge-shaped segments of a large cylindrical vessel. Solvent percolating through the cells falls into the bottom of the extractor housing, where it is picked up by a series of pumps and recirculated countercurrent to the flakes.
Processing Of Extracted Oil
The extent of processing applied to fats depends on their source, quality, and ultimate use. Many fats are used for edible purposes after only a single processing step—i.e., clarification by settling or filtering. Most cold-pressed oils (for example, cold-pressed olive, peanut, and some coconut and sunflower oils) can be used in food products without further processing. Tremendous quantities of butter and lard are used without special treatment after churning or rendering. The growing demand for bland-tasting and stable salad oils and shortening, however, has led to extensive processing techniques. (See Figure 1.)
The nonglyceride components contribute practically all the colour and flavour to fats. In addition, such materials as the free fatty acids, waxes, colour bodies, mucilaginous materials, phospholipids, carotenoids, and gossypol (a yellow pigment found only in cottonseed oil) contribute other undesirable properties in fats used for edible and, to some extent, industrial purposes.
Alkali refining
Many of these can be removed by treating fats at 40° to 85° C (104° to 185° F) with an aqueous solution of caustic soda (sodium hydroxide) or soda ash (sodium carbonate). The refining may be done in a tank (in which case it is called batch or tank refining) or in a continuous system. In batch refining, the aqueous emulsion of soaps formed from free fatty acids, along with other impurities (soapstock), settles to the bottom and is drawn off. In the continuous system the emulsion is separated with centrifuges. After the fat has been refined, it is usually washed with water to remove traces of alkali and soapstock. Oils that have been refined with soda ash or ammonia generally require a light re-refining with caustic soda to improve colour. After water washing, the oil may be dried by heating in a vacuum or by filtering through a dry filter-aid material. The refined oil may be used for industrial purposes or may be processed further to edible oils. Usually, the refined oils are neutral (i.e., neither acidic nor alkaline), free of material that separates on heating (break material), lighter in colour, less viscous, and more susceptible to rancidity.
Water refining
Water refining, usually called degumming, consists of treating the natural oil with a small amount of water, followed by centrifugal separation. The process is applied to many oils that contain phospholipids in significant amounts. Since the separated phospholipids are rather waxy or gummy solids, the term degumming was quite naturally applied to the separation. The separated phospholipid emulsion layer from oils such as corn (maize) and soybean oils may be dried (commercially, these products are called lecithin) and used as emulsifiers in such products as margarine, chocolate products, and emulsion paints. The degumming of crude soybean oil, which has an average phospholipid content of 1.8 percent, provides the primary source of commercial lecithin. To obtain products of lighter colour, hydrogen peroxide may be added as a bleaching agent during the drying of lecithin. The degummed oil may be used directly in industrial applications, such as in paints or alkyd resins, or refined with alkalies for ultimate edible consumption.
Bleaching
If further colour removal is desired, the fat may be treated with various bleaching agents. Heated oils are treated with fuller’s earth (a natural earthy material that will decolorize oils), activated carbon, or activated clays. Many impurities, including chlorophyll and carotenoid pigments, are adsorbed onto such agents and removed by filtration. Bleaching often reduces the resistance of oils to rancidity, because some natural antioxidants are removed together with impurities. When many oils are heated to more than 175° C (347° F), a phenomenon known as heat bleaching takes place. Apparently the heat decomposes some pigments, such as the carotenoids, and converts them to colourless materials.
Destearinating or winterizing
It is often desirable to remove the traces of waxes (e.g., cuticle wax from seed coats) and the higher-melting glycerides from fats. Waxes can generally be removed by rapid chilling and filtering. Separation of high-melting glycerides, or stearine, usually requires very slow cooling in order to form crystals that are large enough to be removed by filtration or centrifuging. Thus linseed oil may be winterized to remove traces of waxes that otherwise interfere with its use in paints and varnishes. Stearine may be removed from fish oils in order to separate the solid glycerides that would detract from its use in paints and alkyd resins. At the same time, fish stearine is more suitable than whole oil for edible purposes. Cottonseed and peanut oils may be destearinated to produce salad oils that remain liquid at low temperatures. Tallows and other animal fats may be destearinated for simultaneous production of hard fats (high in stearic acid content for special uses such as in making candles) and of liquid oil called oleo oil.
Hydrogenation
For many edible purposes and for some commercial applications it is desirable to produce solid fats. Many shortenings and margarines contain hydrogenated (hardened) oils as their major ingredients. The development of margarine and shortening products resulted from the invention of a successful method for converting low-melting unsaturated fatty acids and glycerides to higher-melting saturated products. The process consists of the addition of hydrogen in the presence of a catalyst to the double (unsaturated) bonds. Thus oleic or linoleic acid (or their acid radicals in glycerides), which are normally liquid at room temperature, can be converted to stearic acid or the acid radical by the addition of hydrogen.
Limited use was made of this hydrogenation technology in Europe; the greatest potential use for the process lay in the United States, where a vast production of cottonseed oil, a by-product of the Southern cotton industry, awaited developments that would permit its conversion to a plastic fat. The hardening of cottonseed oil in the early 1900s gave birth to the shortening industry. Practical hydrogenation then spread to all countries where margarines and shortenings are produced from liquid oils.
Hydrogenation reactions
In commercial practice, hydrogenation is usually carried out with vigorous agitation or hydrogen dispersion with a narrow range of catalyst concentration (about 0.05 to 0.10 percent of finely divided nickel suspended on kieselguhr, or diatomaceous earth) in a steel pressure-reaction vessel. The ordinary ranges of temperature and pressure are from 100° to 200° C (212° to 392° F) and from atmospheric pressure to 42 kilograms per square centimetre, respectively. These conditions can be controlled to make the hydrogenation reaction somewhat selective—i.e., to add hydrogen to the linolenic (three double bonds) and linoleic (two double bonds) acid radicals before adding to the oleic (one double bond) acid radicals. The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. Copper-containing catalysts are especially selective in the hydrogenation of vegetable oils. If very hard fats with low amounts of unsaturation are desired and selectivity is unimportant, higher temperatures and pressures are employed to shorten the reaction time and to use partially spent catalyst that would otherwise be wasted. After hydrogenation, the hot oil is filtered to remove the metallic catalyst for either reuse or recovery.
Isomerization reactions
During the catalytic treatment another reaction also takes place—isomerization (rearrangement of the molecular structure) of unsaturated fatty acid radicals to form isooleic, isolinoleic, and similar groups. Because these isomers have higher melting points than do the natural acids, they contribute to the hardening effect. The unsaturation of natural oils has the cis configuration, in which hydrogen atoms lie on one side of a plane cutting through the double bond and alkyl groups lie on the other side. During hydrogenation some of the unsaturation is converted to the trans configuration, with like groups on opposite sides of the plane. The trans isomers are much higher melting than the natural cis form. Simultaneously with the change of some of the unsaturation to the trans configuration there is a migration of double bonds along the chain. Thus isomers of oleic acid may be formed with the double bond in any position from carbon atom 2 to carbon atom 17. Many of these isomerized acids are higher melting than the natural oleic acid. Infrared analysis is useful for quantitative measurement of changes occurring during hydrogenation.
Deodorization
Odourless and tasteless fats first came into high demand as ingredients for the manufacture of margarine, a product designed to duplicate the flavour and texture of butter. Most fats, even after refining, have characteristic flavours and odours, and vegetable fats especially have a relatively strong taste that is foreign to that of butter. The deodorization process consists of blowing steam through heated fat held under a high vacuum. Small quantities of volatile components, responsible for tastes and odours, distill, leaving a neutral, virtually odourless fat that is suitable for the manufacture of bland shortening or delicately flavoured margarine. Originally, deodorization was a batch process, but increasingly, continuous systems are being used in which hot fat flows through an evacuated column countercurrent to the upward passage of steam. In Europe, a deodorization temperature of 175°–205° C (347°–401° F) is common, but in the United States, higher temperatures of 235°–250° C (455°–483° F) are usually employed. About 0.01 percent of citric acid is commonly added to deodorized oils to inactivate trace-metal contaminants such as soluble iron or copper compounds that otherwise would promote oxidation and the development of rancidity.
Olive oil is invariably marketed in undeodorized form. The natural flavour is an important asset, and olive oil, as is true of butter, commands a premium in the market because of its distinctive and prized flavour. The common cooking oils of Asia—soybean, rapeseed, peanut, sesame, and coconut—are consumed in their crude form as expressed from oilseeds. In contrast, deodorized oils are in particular demand in the United States and Europe. For many years the only important vegetable oil consumed in the United States was cottonseed oil, which in its crude form has such a strong and unpleasant flavour that further processing was an absolute necessity in order to render it suitable for consumption. Because of widespread sale of neutral-flavoured cottonseed oil products over many years, a general preference was developed for odourless and tasteless fats.
Another reason for the practice of deodorizing edible oils in Europe and America relates to differences in oil quality by Western and Eastern extraction techniques. In China and Southeast Asia, edible oils have been produced principally by small, relatively crude equipment. The yield of oil is relatively low, and a minimum amount of nonglyceride substances is expressed from the seed, with the result that the flavour of the oil is fairly mild. In Europe and the United States, oil extraction is carried out in large factories that operate on an extremely competitive basis. Very-high-pressure expression or solvent extraction is used, and in order to improve yields the seeds are heat-treated prior to extraction. Oils obtained in high yield under such conditions are stronger in flavour than oils prepared by low-pressure expression, and the refining and deodorizing steps are required to improve palatability. The improvement in yields more than compensates for the added costs of refining and deodorizing.
When fats are hydrogenated for manufacture of margarine and shortening, they develop a characteristic sweet, but rather unpleasant, “hydrogenation odour” that must be removed from edible fats by deodorization.


